





Research in the Brumaghim Group
|
Research in the Brumaghim group aims to understand how metal ions form radical species that damage DNA and how antioxidants prevent this damage. Work in our group can be divided into three main categories: DNA damage and prevention, antioxidant coordination chemistry, and cell death prevention in E. coli and mammalian cells. Studying mechanisms for DNA damage and cell death inhibition by antioxidants may lead to treatments for a wide range of chronic diseases, including cardiovascular diseases, Alzheimer's and Parkinson's diseases, cancer, and diabetes. It could also help prevent radiation damage during radiation treatments and long-duration space flight. Antioxidants prevent DNA damage
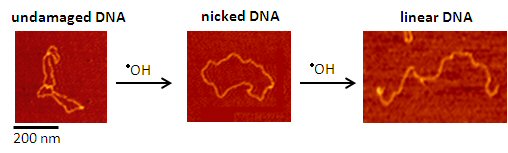
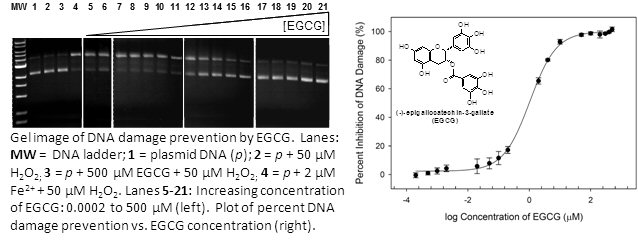
Undamaged DNA is separated from damaged (nicked and linear) DNA using gel electrophoresis. Adding an antioxidant prevents DNA damage from metal-generated •OH. We quantify the amount of DNA damage inhibition seen in the gels and directly compare the effectiveness of different antioxidants.
We discovered that sulfur, selenium, and polyphenol antioxidants prevent metal-mediated DNA damage through metal coordination. Currently we are developing structure-activity relationships for prevention of DNA damage by each of these classes of antioxidants. For example, the ability of polyphenol antioxidants to prevent iron-mediated DNA damage can be predicted simply from the pKa of the first phenolic hydrogen atom. This is because the polyphenol groups must be deprotonated to bind iron. Understanding this novel metal-binding mechanism of antioxidant activity will aid the design of more potent antioxidant compounds. We are also currently investigating the interactions of both antioxidants and metal ions with DNA. Biological coordination chemistry
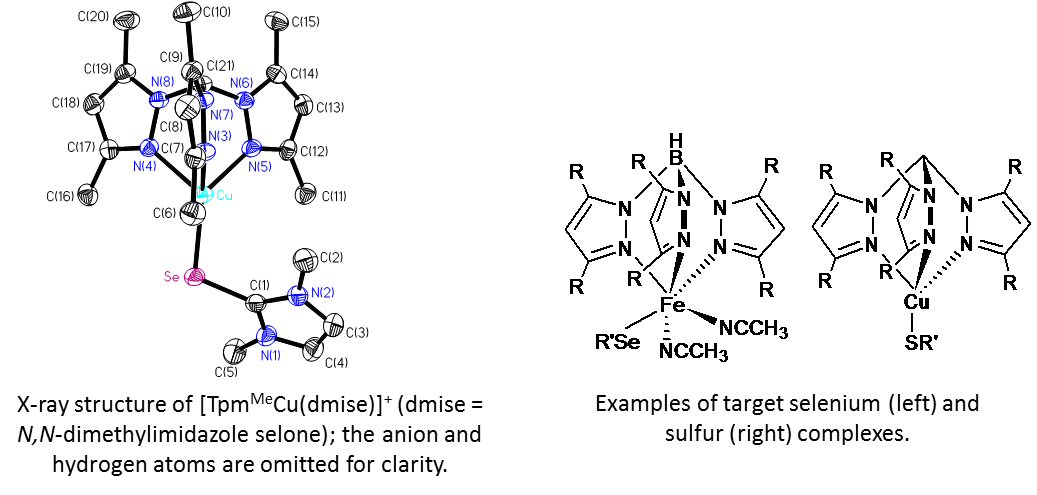
To synthesize our target selenium and sulfur compounds, we use TpR and TpmR (tris(3,5-R- pyrazolyl)borate and -methane, respectively) and selone, thione, or selenoamino acid ligands with Cu(I/II) or Fe(II/III). We are currently synthesizing second-generation sulfur and selenium antioxidants for testing in addition to synthesizing and characterizing our target complexes by NMR, X-ray crystallography, and cyclic voltammetry. Antioxidants prevent cell death |

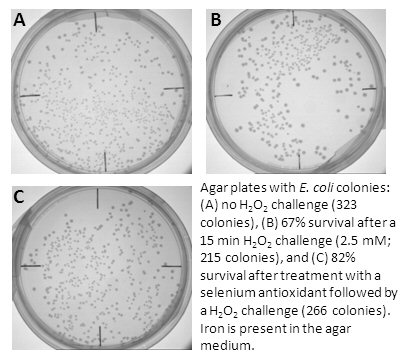 Because we found that antioxidants prevent DNA damage in vitro by metal coordination,
we are also interested in testing antioxidant ability to prevent cell death under oxidative stress.
When cells (both bacterial and mammalian) are exposed to H2O2 or hypoxic conditions,
many die due to the DNA damage caused by metal-generated hydroxyl radical.
Because we found that antioxidants prevent DNA damage in vitro by metal coordination,
we are also interested in testing antioxidant ability to prevent cell death under oxidative stress.
When cells (both bacterial and mammalian) are exposed to H2O2 or hypoxic conditions,
many die due to the DNA damage caused by metal-generated hydroxyl radical.