

 Clemson Bioengineering annually sponsors the Biomedical Engineering and Surgical Technology (BEST) Design Workshop, where stakeholders evaluate how the design of medical-device prototypes may affect patient safety. The 2017 event coordinator was Dr. Melinda Harman, Director of GreenMD, Clemson’s medical device recycling, and reprocessing certificate program.
Clemson Bioengineering annually sponsors the Biomedical Engineering and Surgical Technology (BEST) Design Workshop, where stakeholders evaluate how the design of medical-device prototypes may affect patient safety. The 2017 event coordinator was Dr. Melinda Harman, Director of GreenMD, Clemson’s medical device recycling, and reprocessing certificate program.
 One problem with device reuse is that poor design can prevent the effective cleaning and sterilization that the Food and Drug Association requires between patients. Prototype evaluation is one step in preventing patient risk of a debilitating or fatal illness caused by inadequate cleaning and sterilization.
One problem with device reuse is that poor design can prevent the effective cleaning and sterilization that the Food and Drug Association requires between patients. Prototype evaluation is one step in preventing patient risk of a debilitating or fatal illness caused by inadequate cleaning and sterilization.
 Clemson University Biomedical Engineering Innovation Center (CUBEInC), a state-of-the-art facility primarily dedicated to biomedical research and accelerated innovation, was the site of the Workshop.
Clemson University Biomedical Engineering Innovation Center (CUBEInC), a state-of-the-art facility primarily dedicated to biomedical research and accelerated innovation, was the site of the Workshop.
 CUBEInC has 30,000 sq. ft. of research laboratories and education facilities, translational/incubator space, bioimaging research facilities, and state-of-the-art surgical-skills facilities.
CUBEInC has 30,000 sq. ft. of research laboratories and education facilities, translational/incubator space, bioimaging research facilities, and state-of-the-art surgical-skills facilities.
 CUBEInC is the translational research arm of the Clemson University School of Health Research (CUSHR), providing the environment essential to developing clinically driven technology and addressing research questions. Opened in 2011, CUBEInC facilitates student and faculty collaboration with healthcare practitioners.
CUBEInC is the translational research arm of the Clemson University School of Health Research (CUSHR), providing the environment essential to developing clinically driven technology and addressing research questions. Opened in 2011, CUBEInC facilitates student and faculty collaboration with healthcare practitioners.
 The Workshop included an overview of GreenMD from Dr. Harman on medical device recycling and reprocessing. GreenMD is an industry immersion and training program for engineers to adapt medical device designs for sustainability, to impact healthcare safety with green technology, and to connect with medical and industry leaders.
The Workshop included an overview of GreenMD from Dr. Harman on medical device recycling and reprocessing. GreenMD is an industry immersion and training program for engineers to adapt medical device designs for sustainability, to impact healthcare safety with green technology, and to connect with medical and industry leaders.
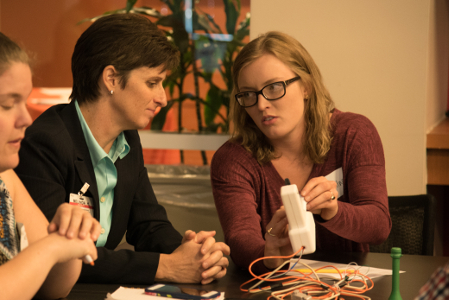 Market research firms agree that the global market for reprocessed medical devices will be measured in billions of dollars within the next decade. Bioengineers are expected to be involved in designing, developing, marketing and regulating such devices.
Market research firms agree that the global market for reprocessed medical devices will be measured in billions of dollars within the next decade. Bioengineers are expected to be involved in designing, developing, marketing and regulating such devices.
 Katie Jurewicz, Ph.D. candidate in industrial engineering, presented the keynote address on the role of human factors in medical device design.
Katie Jurewicz, Ph.D. candidate in industrial engineering, presented the keynote address on the role of human factors in medical device design.
 She described human factors engineering, noting that the FDA requires that knowledge about human behavior, abilities and limitations and other characteristics of medical device users be part of the design process and included when safety and efficacy of medical device use are demonstrated.
She described human factors engineering, noting that the FDA requires that knowledge about human behavior, abilities and limitations and other characteristics of medical device users be part of the design process and included when safety and efficacy of medical device use are demonstrated.
 Pitching their prototypes of medical devices for review, five bioengineering teams made presentations.
Pitching their prototypes of medical devices for review, five bioengineering teams made presentations.
 Attendees completed a reprocessing assessment report for each design to identify characteristics that could help or hinder the device’s reusability.
Attendees completed a reprocessing assessment report for each design to identify characteristics that could help or hinder the device’s reusability.