Compressed Fluids Technologies

Subcritical Fluid Technology:
Metal Desalination for Corrosion Mitigation
Stabilization of iron artifacts is of paramount importance in their long term preservation. In the case of materials recovered from sea or brackish water desalination is the most vital step of this process. As iron corrodes in saline environments the abundant chloride anions present in the water are attracted to the sites were corrosion takes place. These salt-rich sites located throughout cracks and crevices reaching far from the original surface of the object are often found surrounded by layers of corrosion products and marine concretion (mix of corrosion products and materials from the surrounding environment – i.e. sand, shells, micro fauna.). If after recovery, salt-loaded artifacts are allowed to dry in presence of oxygen rapid corrosion takes place with devastating effects to the artifacts. This results in the loss of relevant archaeological information associated with the object and often the complete destruction of the artifact itself.
Up until recently the standard methods of desalination required would have been to simply soak the artifact in an alkaline solution and let the salts gradually seep out from within. This process would normally take several years to decades depending on the artifact (material, size and shape, complexity, state of degradation, etc.) to reduce chloride levels to a safe threshold. Alternatively, a process of electrolysis could be done to help expedite this process, but sometimes this would result in mixed outcomes that could potentially damage the object.
These techniques have their own advantages and disadvantages, particularly in their effectiveness, required time for completion, and levels of risk involved to the artifact in question. Since 2003 conservators, scientists, and engineers at the Warren Lasch Conservation Center in Charleston, SC have been developing a new technique called the subcritical treatment. Altering the temperature and pressure of the water, properties such as viscosity, surface tension, and density will change. By modifying these properties water becomes much more effective in penetrating very small cracks and pores in the iron artifacts and allows the extraction of salts much quicker than water at atmospheric conditions ever could. Subcritical water effectively reduces stabilization time from years to weeks.
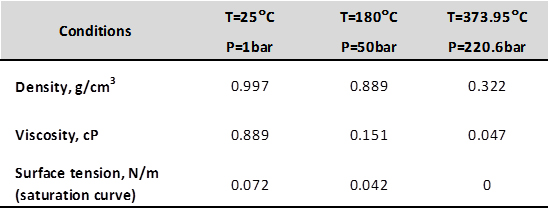
How are the subcritical conditions reached? Starting at room temperature and atmospheric pressure, the last is increased to 50 bars by means of a pump that drives the treatment solution into the reactor’s cell containing the artifacts. The system is then gradually heated to a final temperature of 180°C. For several days sodium hydroxide is passed through the reactor continually removing salts from the materials under treatment. Once the chloride concentration inside the reactor reaches negligible levels the desalination step is considered complete.
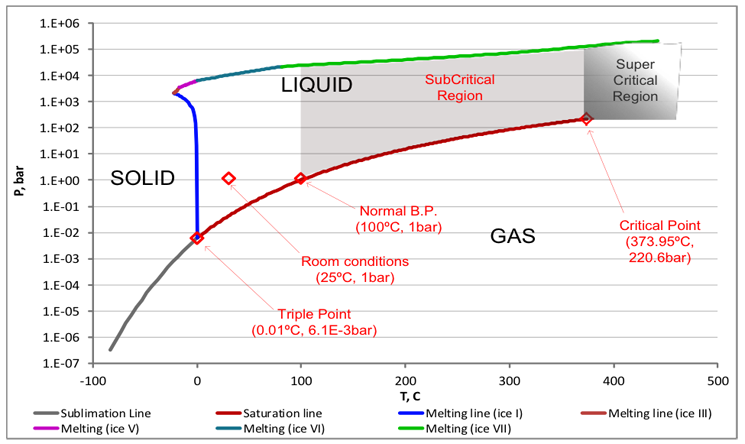
Subcritical water is heated under the appropriate pressures between its atmospheric pressure boiling temperature of 100°C and critical temperature (Tc) of 374°C while still remaining in a liquid state. Water that is heated beyond its critical point of 374°C, and over 220.5 bar is supercritical water.
Prior to this research conducted at the Warren Lasch Conservation Center, there were no reference points to go on in the use of super or subcritical fluids in the stabilization of iron archeological artifacts.

Active Corrosion on Iron Surface

Nut and Bolt before, during, and after subcritical treatment
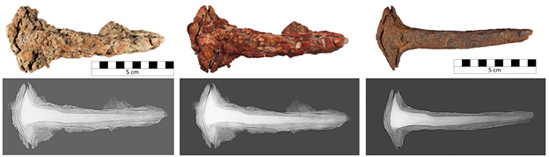
Nail before, during, and after subcritical treatment

T-handle from the H.L. Hunley (courtesy of the Friends of the Hunley)
Supercritical Fluids Technology – CO2
Examples of Industrial Uses:
- Supercritical Fluid Extraction, Separation and Chromatography
- Catalysis and Polymerization
- Injection molding & Extrusion
- Particle Formation (coatings, pharmaceuticals)
- Cleaning & Dyeing
- Plastic Production and Recycling
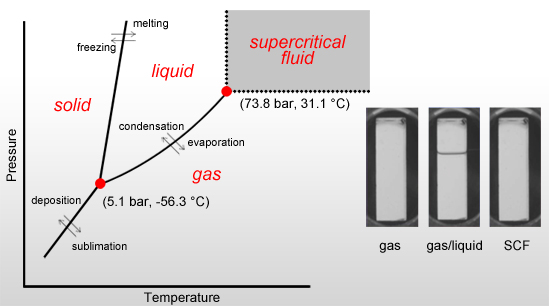
Supercritical CO2 Drying Process
Archaeological waterlogged cork is one the most unpredictable archaeological materials to conserve. Over the years, various techniques designed to conserve waterlogged wood have been applied to cork with less than satisfactory results. The Warren Lasch Conservation Center (WLCC) has shown successful conservation of significant archeological corks and composite artifacts from several shipwrecks (Machault (1760), San Juan (1565), Queen Anne’s Revenge (1718), H.L. Hunley (1864)) using supercritical CO2.


