Publication Articles
Africanized Honey Bee Q&A
This update is a list of frequently asked questions about the Africanized Honey Bee. Clemson University Cooperative Extension Service and the U.S. Department of Agriculture have prepared this list of questions and answers to help educate the public on the Africanized Honey Bee.
Question: Are Africanized honey bees the same frightening "killer bees" I've read about and seen on the news and in movies?
Answer: Yes and no. The two names refer to the same bee, but the correct name is Africanized Honey Bee (AHB). The AHB is the hybrid offspring of African honey bees brought to Brazil in 1956 and honey bees commonly found in this hemisphere. Because of the way AHBs have been portrayed in the media, some people believe these bees swoop down from the sky, en masse, causing death and destruction. This is not true. In reality, the chances of being injured by an Africanized or any honey bee are far less than the chances of being hit by lightning.
Question: When did the AHB enter the U.S. and what states have AHBs?
Answer: The first known AHBs to enter the U.S. from Mexico occurred in the Rio Grande River Valley near Hidalgo, Texas, on October 15, 1990. The AHBs have now spread mostly westward in the U.S. and are now found in Arizona, California, Nevada, New Mexico, Puerto Rico, and Texas.
Question: Will the Africanized Honey Bee move into South Carolina?
Answer: Nobody knows for sure, but some scientists believe they will survive only in the southern United States where winters are relatively mild. Our Coastal and Pee Dee areas of South Carolina are within this mild winter range. Other bee scientists predict the AHB will survive wherever other honey bees are kept which includes all of South Carolina.
Question: How fast do AHBs spread?
Answer: AHBs spread through South and Central America at a rate of 100 to 300 miles per year. The bees have continued a rapid spread through the extreme southwestern U.S., but their easterly spread has been much slower and they have not advanced east of Texas. This phenomenon has scientists perplexed looking for an explanation for the stall in the eastward advance of the AHB in the U.S. This could be a temporary halt in the advance eastward or the AHB may have reached their northern limit of range expansion into North America.
Question: Is one sting from an AHB deadly?
Answer: One sting from an AHB bee is no more painful or dangerous than a sting from any other honey bee, but AHBs tend to sting with less provocation and in greater numbers than other honey bees. It is important to remember that one sting from any bee can be harmful, even fatal, if the person stung is allergic to bee venom. People allergic to bee venom should always carry a first-aid sting kit with them. The best way to keep from being stung is to stay away from places where the bees live, including managed and wild colonies.
Question: What makes AHBs and other bees sting?
Answer: Honey bees sting when their nest is threatened by invaders. AHBs are different from our local honey bees in that they tend to defend their hives more quickly and in greater numbers. Generally, Africanized honey bees pursue intruders further than other honey bees do.
Question: How many stings will Africanized honey bees inflict if their nest is threatened?
Answer: Like all honey bees, AHBs sting only once because they die shortly after stinging. However, as a group, AHBs tend to inflict more stings than domestic honey bees because they react in greater numbers. One study shows that, in similar situations, Africanized honey bees have stinging responses ten times greater than that of the domestic honey bees. Whatever the number of stings, AHBs can be very serious pests and must be treated with caution.
Question: Have human deaths occurred in the U.S. as a result of AHB stings?
Answer: Yes, between 1993 and 1999, six deaths have been attributed to AHBs in the U.S. These cases have mostly involved elderly adults who could not escape and received multiple stings.
Question: What does an Africanized honey bee look like?
Answer: To the naked eye, an AHB looks just like any other honey bee. The only way to distinguish between Africanized and other honey bees is by microscopic examination. Honey bees are brown or black and covered with hair.
Question: How fast does an Africanized Honey Bee fly?
Answer: About the same as any honey bee, about 12-15 miles an hour.
Question: How far will an AHB chase an intruder?
Answer: Some have been known to fly as far as a quarter mile, but they usually stop if they can't detect an intruder or if there is no longer a threat to their colony.
Question: How can I stay away from an AHB swarm and avoid getting stung?
Answer: People unfamiliar with beekeeping should always stay away from all bees and swarms, regardless of the kind of bee. If you see a swarm or hive of bees, regardless of the kind of bees inside, it is best just to walk away.
Question: Will a swarm chase me?
Answer: Because swarms are not established colonies with hives to defend, most will not chase you. Most bees, including the AHB, are not particularly defensive if they don't have a home to defend.
Question: What is being done in South Carolina to prepare for the arrival of the Africanized Honey Bee?
Answer: A South Carolina Africanized Honey Bee Advisory Committee was formed in April 1989. The committee has formulated a State Africanized Honey Bee Management Plan.
Reference:
James Tew, June 1990, "Frequently asked questions about the Africanized Honey Bee", USDA Backgrounder, USDA, Washington, D.C.
Prepared by Wm. Michael Hood, Extension Entomologist/ Professor, Department of Entomology, Soils, and Plant Sciences, Clemson University.
Providing Leadership in Environmental Entomology
Department of Entomology, Soils, and Plant Sciences, 114 Long Hall, Clemson, SC, 29634-0315, 864-656-3111
This information is supplied with the understanding that no discrimination is intended and no endorsement by the Clemson University Cooperative Extension Service is implied. Brand names of pesticides are given as a convenience and are neither an endorsement nor guarantee of the product nor a suggestion that similar products are not effective. Use pesticides only according to the directions on the label. Follow all directions, precautions and restrictions that are listed. EIIS/AP-8 (New 01/2002).
Small Hive Beetle
Scientific Name: Aethina tumida
Family: Nitidulidae - Sap Beetles
In July 1998, small hive beetles were first identified in South Carolina. The beetles were collected from a weak honey bee colony in Charleston County. The small hive beetle has also been discovered in Florida and Georgia in May and June 1998, respectively. Prior to these discoveries, the beetle had not been reported to infest beehives except in tropical and sub-tropical regions of South Africa where it is considered to be a secondary hive pest.

Worker Honey bee and adult small hive beetle.
The small hive beetle has been confirmed in 16 South Carolina counties including Bamberg, Barnwell, Berkeley, Beaufort, Charleston, Colleton, Dorchester, Florence, Hampton, Jasper, Lexington, Oconee, Orangeburg, Pickens, Sumter and Richland. The discovery of the beetle over such a large area indicates the beetle has been present in our state for several years. The initial point of entry of this pest into our state is unknown, however recent investigations of unidentified beetle specimens that were submitted in November 1996 have yielded positive identification of the small hive beetle. The sample of beetles were collected from a managed honey bee colony in Charleston County. Another unidentified sample of the small hive beetle was submitted from Berkeley county in 1997. These earlier beetle samples were identified to family name only because no means of identifying the pest to genus and species were available at that time and no one suspected the small hive beetle to be found in North America.
The small hive beetle is not considered a serious problem in South Africa where the pest only damages weak bee colonies and stored comb. Apis mellifera scutellata, the African honey bee, apparently does a better job of controlling this pest than our European honey bees. Improved hygienic behavior by the Africanized honey bee and the habit of quickly absconding under light predation likely play a major role in keeping the beetles in check in South Africa.
Identification:

Worker Honey bee and adult small hive beetle.
The adult beetles have 6 legs and 2 pair of wings, are reddish brown to black in color, and are about 5-7 mm (about ¼ inch) long. The dorsal side or back of the beetle has very fine hairs which cause the fast moving insect to be very slippery and difficult to grasp and remove from the colony. The exterior of the adult beetle is very hard and protects the beetle from bee stings and other dangers. Adult beetles fly readily, but their flight range is unknown.
Small hive beetle eggs are pearly white and look much like honey bee eggs, but they are slightly smaller. Eggs are expected to be laid throughout the year in South Carolina except during the colder months of December - March.
Larvae are cream colored and when fully grown are about 11 mm (7/16 inch) long and slightly more than 1.6 mm (1/16 inch) in diameter. The larvae look much like young wax moth larvae, but never reach the size of mature wax moth larvae. Small hive beetles and wax moths can infest the same honey bee colony. The small hive beetle larvae have only 3 pair of prolegs which are located near the head, whereas the wax moth larvae have many small, uniform pairs of legs along the body. Beetle larvae do not spin a cocoon as do wax moth larvae. Beetle larvae have tough exterior bodies; wax moth larvae have soft exterior bodies that can be penetrated easily.

Small hive beetle larvae on comb
Pupae are light tan to blueish tan in color and are approximately the same size as the adult beetle. The mature pupae have the same appendages as the adult beetle, but are undeveloped. Life Cycle: Female adult beetles prefer to deposit irregular masses of eggs in crevices or cavities inside the hive, although eggs are often found in comb not defended by bees. Studies in South Africa indicate that eggs hatch in 2-3 days. The females are thought to have a great reproductive capacity and a few fertile females can result in a heavy infestation in a single generation.
Beetle larvae mature from egg hatch to pupae in 10-16 days. Larvae require a diet of honey and pollen to develop fully. The mature larvae leave the hive and burrow into the soil beneath the hive to pupate. The length of the pupal stage varies, but most pupae emerge from the soil as adults in 3-4 weeks.
Newly emerged adults are very active and fly readily when disturbed; the newly emerged adults reenter a bee colony and begin the life cycle again. Female beetles begin laying eggs about one week following their emergence from the soil. As the adults age, they seek refuge and seldom fly. The adults normally gather at the rear of the hive on the hive floor where they feed on pollen that falls from the brood area above. Adult beetles are often seen in the top of the hive as the beekeeper removes the inner cover. Adults live up to 6 months, therefore more than one generation may live in the same bee colony. Five beetle generations per calendar year are possible in South Africa.
Economic Importance:
The South Carolina beekeeping industry faces another threat, the small hive beetle, in addition to the tracheal and varroa mites that have been a major problem over the past 10 years. Early reports of damage caused by this beetle in the US indicate that the small hive beetle may be a more virulent pest than in South Africa where it is known only as a secondary hive problem. Reports indicate that when bee colonies do not readily eject the beetles from the hive, strong as well as weak colonies may be equally affected.
Small hive beetle adults and larvae cause much damage inside the hive. Feeding larvae consume pollen and honey and heavily damage wax comb, especially newly drawn comb. Old sturdy comb can better withstand heavy larval feeding. Honey leaks from the damaged comb and is normally spoiled from beetle waste, producing a frothy mess; the resulting fermented honey is useless to the bees which may abandon the affected equipment and sometimes abscond.
Beetle larvae also feed on stored comb and supers of honey in honey houses, especially frames containing pollen. The beetle is a major problem when honey supers are allowed to remain in the honey house for long periods prior to extraction. Wax cappings set aside following honey extraction may become beetle infested, also. Bee brood rearing stops when the beetle infestation reaches high levels in a colony and reports indicate that the colony will not be able to overcome the problem.
Beekeeping management costs in South Carolina will increase due to the negative effects caused by the small hive beetle. Extra costs include: 1) time and labor to detect and treat for the beetles; 2) treatment materials; 3) colony replacement from beetle losses; 4) damaged equipment replacement; 5) loss in honey production and pollination from affected colonies; 6) income loss from pollination rentals and honey sales.
Prevention:
Maintaining healthy bee colonies that are capable of protecting all comb in the hive is desirable in preventing small hive beetle problems. Mite control and disease prevention along with keeping a young laying queen are important. Removing excess supers of unprotected honey and empty comb from the hive is highly recommended.
Beekeepers should avoid leaving filled honey supers standing in the honey house for more than a day or so. Stacking lightly infested beetle supers on strong bee colonies for clean up as for wax moth control should not be practiced. This only spreads the beetles and may result in loss of additional colonies.
Purchasing colonies, packages, and nucs from reliable sources that have been certified beetle free is highly recommended. The beekeeper should make a thorough inspection of colonies prior to making splits or exchanging brood frames between colonies to prevent spreading the beetles. Young colonies are particularly vulnerable to beetle problems, so every attempt should be made to maintain healthy colony conditions.
Detection:
Beekeepers should inspect their colonies for signs of small hive beetle infestations. Adult beetles run across comb seeking refuge when the hive is first opened. Beetle adults are often discovered underneath the hive top and cover or at the rear area of the bottom board. In a heavily infested colony, beetle adults and larvae or grubs are found seeking shelter under comb debris on the bottom board. Beetle larvae can also be found in comb cells and in the soil beneath the hive along with pupae.
Beekeepers should suspect small hive beetle activity when fermented honey runs out the hive entrance or pools on the honey house floor. A “decaying orange” odor is often given off by the spoiled honey. A colony heavily infested with beetles may result in many worker bees massing at the hive entrance as they abandon beetle infested areas of the colony. Another symptom of a heavily infested colony includes the appearance of wet or greasy bees and frames. Night detection of small hive beetles on hive frames is possible when amber light is directed inside the colony. Varroa mite sticky boards have proven ineffective in beetle detection as the adult beetles move easily across the sticky material. Even a heavy coating of a much stickier insect trapping material such as Tangle Foot® has proven to be ineffective in stopping adult beetles.
Small card board inserts with one side of the card board surface removed have been successful in beetle surveys. The insert should be placed at the rear of the hive on the bottom board where adults normally gather. The adult beetles are apparently attracted to the insert and seek shelter in the corrugations.
"Preventive" soil treatments for this pest are not recommended especially in areas free of the beetle.
If small hive beetles are suspected in counties outside known infested areas, the beekeeper should place a few of the beetles, adults or larvae, in a small bottle of alcohol and deliver the specimens to the local Clemson University Cooperative Extension Office for Problem Clinic processing. Or, the beekeeper may forward specimens to Department of Plant Industry apiary inspector Fred Singleton, 204-B North Gum Street, Summerville, SC. 29483, Phone: (843) 821-3234, Email: fsngltn@clemson.edu.
Control:
Once a beekeeper detects the small hive beetle, he/she should manage all colonies with diligence to prevent stressful conditions that might enhance beetle activity. Check Mite +®, formerly named Bayer Bee Strip, is registered in SC as an inside - hive treatment for small hive beetle control. Colony treatment with this product must be applied at a time when bees are not producing a surplus honey crop. Remove honey supers before application of Check Mite + strips and do not replace until 14 days after the strips are removed. The production and sale of comb honey is prohibited in colonies treated with this product. Follow label directions for all pesticides. Use this product only when outside temperature is 70°F (21°C) or greater.
A control measure for dead colonies includes freezing the hive and its contents at 10°F (-12°C) for 24 hours. This will kill all life stages of the beetle. Equipment should be checked for beetle activity upon removal from the freezer especially in hot weather when longer periods of freeze time are sometimes necessary.
Honey bees will not normally accept supers that are contaminated with beetle fermented honey. The fermented honey can be washed off with a garden hose and bees will normally rework the equipment.
A vulnerable point of the beetles’ life cycle is when mature larvae enter the soil underneath the hive to pupate. Gard Star® is registered as a soil drench pesticide to control small hive beetles. Direct pesticide bee contact or indirect chemical fumes may cause massive bee kills, therefore placement of colonies on hive stands are highly recommended. Beekeepers should read the product label carefully and follow all directions. their potential negative impacts to the bees can be minimized by making soil pesticide applications just before dusk to avoid peak bee flight activity and to allow the pesticide to dry before next day.
On the other hand, natural insect predators such the imported fire ant, which is now found in every South Carolina county, may feed on beetle pupae in the soil. High density fire ant mounds of 100 mounds per acre (250 mounds per hectare) may be required for effective control, however the predation level may be dependent on the availability of other food sources on the site. Hive equipment that is heavily infested with beetles may be placed over a large fire ant mound for cleanup.
Beekeepers should not experiment with illegal pesticide treatments for beetle control. The beekeeping industry must rely on bee scientists who make investigations under controlled conditions. Remember that most materials that will kill the small hive beetle will also kill honey bees or worse may injure the beekeeper and contaminate honey and the equipment.
References:
- Caron, D.M. 1997. Other insects. In Honey bee pests, predators and disease. 3d ed (R.A. Morse & K. Flottum eds.) A.I. Root Co., Medina, Ohio.
- Delaplane, K.S. 1998. The small hive beetle, Aethina tumida - A new beekeeping pest. Bugwood 98. University of Georgia.
- Fore, T.H. 1998. Destructive beetles found in Florida hives. The Speedy Bee. Vol 27:5, pp 1, 6-8.
- Fore, T.H. 1998. Hive beetles still limited, officially, to three states. The Speedy Bee. Vol 27:7, p. 2.
- Lundie, A.E. 1940. The small hive beetle Aethina tumida. South Africa Department of Agriculture & Forestry Entomological Series 3, Science Bulletin 220.
- McCaskill, V.H. 1998. Personal communications.
- Sanford, M.T. 1998. Aethina tumida: a new beehive pest in the western hemisphere. Apis 16(7), University of Florida.
- Sanford, M.T. 1998. Update on the small hive beetle. Apis 16(8), University of Florida.
- Singleton, F.D. 1998. Personal communications.
Prepared by William Michael Hood, Extension Entomologist/ Associate Professor, Department of Entomology, Soils, and Plant Sciences, Clemson University.
Providing Leadership in Environmental Entomology
Department of Entomology, Soils, and Plant Sciences, 114 Long Hall, Clemson, SC, 29634-0315, 864-656-3111
This information is supplied with the understanding that no discrimination is intended and no endorsement by the Clemson University Cooperative Extension Service is implied. Brand names of pesticides are given as a convenience and are neither an endorsement nor guarantee of the product nor a suggestion that similar products are not effective. Use pesticides only according to the directions on the label. Follow all directions, precautions and restrictions that are listed. EIIS/AP-2 (Revised 01/2000).
Cicada Killer Wasp
In mid- to late summer people begin to encounter large wasps with distinctive bands of white and yellow on black bodies. The head and thorax are a rusty red and the wings are tinged with yellow. The body is about 1.25 to 1.5 inches long. The wing span approaches three inches. Their large size and habit of buzzing around a person terrifies many people.
The female wasps are equipped with a stinger and if seriously provoked can produce a painful sting. The females are territorial and will investigate any thing or anyone coming near their nest burrow. If a person stands still, the female will usually go on about her business.

A cicada killer wasp with an annual cicada
When cicada killers first emerge, the males are very territorial and will fight with each other while establishing territories. Once females begin to emerge the males will attempt to capture them and mate. The males will make every attempt to defend their territory against any invader, including people. The males do not have a stinger and are harmless. However, they can terrify anyone who is afraid of bees and wasps.
Cicada killers are solitary wasps. This means that each female has her own burrow. The burrows are dug in well drained sites. They prefer sandy or bare soils, but will also construct burrows in areas where the grass is kept very short. They can be a real problem in playgrounds and parks where sand has been installed for sports or around playground equipment. They are also a problem around sand traps, tees, and greens on golf courses.
Cicada killers hunt annual or dogday cicadas in trees. When a cicada is spotted, the wasp zooms in and attempts to sting the cicada. The sting paralyzes the cicada. The cicada will utter a buzzing shriek until it becomes paralyzed. Once the cicada becomes motionless, the wasp will carry the cicada to a high point and attempt to fly back to her burrow. While larger wasps may be successful on the first attempt, smaller wasps may need to repeat the process several times.

A cicada killer burrow in a lawn
At the burrow, the wasp places the cicada in a side chamber and lays an egg on it. The egg hatches, and the wasp larva feeds on the body fluids of the paralyzed cicada. Development is completed within two weeks and the wasp larva forms a cocoon. The winter is spent in the larval stage and pupates the following spring. Adult emergence coincides with the emergence of annual cicadas.
In most cases, control of this wasp is not needed. When the burrows are in an area where there is a high risk of someone getting stung, control may be needed. The best approach is to treat the individual burrows with an insecticide formulated as a dust. As the wasp enters or leaves the hole the dust will adhere to her body. As she attempts to clean her body, some of the insecticide will be picked up and it will kill the wasp.
Prepared by Clyde S. Gorsuch, Extension Entomologist/ Professor, Department of Entomology, Soils, and Plant Sciences, Clemson University
Providing Leadership in Environmental Entomology
Department of Entomology, Soils, and Plant Sciences, 114 Long Hall, Clemson, SC, 29634-0315, 864-656-3111
This information is supplied with the understanding that no discrimination is intended and no endorsement by the Clemson University Cooperative Extension Service is implied. Brand names of pesticides are given as a convenience and are neither an endorsement nor guarantee of the product nor a suggestion that similar products are not effective. Use pesticides only according to the directions on the label. Follow all directions, precautions and restrictions that are listed. EIIS/TO-15 (New 08/2003).
Honey Bee Colony Removal From Structures
The honey bee, Apis mellifera (L.), is our most beneficial insect. We must do all we can to protect bee colonies which are pollinators for many of our home and commercially grown fruits and vegetables. The annual contribution of honey bees to food production in the United States is estimated to be $10 billion. Honey bees are important as primary pollinators of almonds, apples, blackberries, blueberries, cantaloupes, cranberries, cucumbers, forage crops, kiwi fruit, squash, and watermelons. Annual cash receipts of South Carolina commercially grown apples, cantaloupes, cucumbers, and watermelons are estimated at $25 million.
In South Carolina, there are about 2,500 beekeepers who keep bees for pleasure and profit. They manage about 30,000 honey bee colonies which produce 1.2 million pounds of surplus honey annually in addition to the pollination service which many people take for granted.
As good citizens, we should do everything possible to salvage honey bee colonies that become a problem in structural walls, but there are many instances when this is not possible. If a local beekeeper cannot be found to take the bees, the property owner has every right to treat honey bees as pests, especially when human health is threatened. Some basic knowledge of honey bees and their activities is necessary before attempting to remove honey bee colonies from structures:
- Honey bees will defend themselves if threatened. Avoid disturbing a bee colony unless you are fully protected. If you encounter bees that are flying and appear to be out of control, simply walk away slowly without swatting at the bees. Swatting the bees only irritates them more. If you find yourself being attacked by defensive bees, cover your head with your shirt and run quickly through dense vegetation or seek shelter in a vehicle or building.
- The guard or worker honey bees have a barbed stinger that is left behind in the victim. In the process of stinging, a honey bee rips a portion of her abdomen away with the stinger and the bee dies soon. The stinger should be removed immediately from the victim to reduce the amount of venom entering the sting site. A preferred sting remedy is to place a mound of common table salt on the point of sting entry and dampen with water. By osmotic pressure, most of the venom will be withdrawn from the body tissue if the treatment is applied within 3-4 minutes following the stinging incident. When working outside, it is advisable to carry small amounts of salt such as the packets found at fast food restaurants.
- Honey bee swarming season normally occurs in South Carolina during the months of May and June, although there are exceptions. This is the time of year when swarms may enter the wall of structures and become a pest problem. A swarm will enter a void or cavity that averages about 10 gallons (40 liters) in size. Normally, honey bees favor a cavity entrance that faces southeast and is about 1.5 inches (38 mm) in diameter.
- A mature honey bee colony can range in population size of 20,000 to 100,000 bees depending on the season. The colony population will peak from late spring to summer and reach a low point in winter.
- A European honey bee colony will swarm normally once a year whereas an Africanized colony will swarm several times annually. Swarms occur in spring during strong nectar flows when the colony population outgrows its living quarters. The old queen and about half the bees will emerge from the parent colony to find a new home. The parent colony has made preparations to replace the old queen prior to swarm emergence. When the swarm emerges, the bees will cluster on a nearby tree limb or other object and remain there from an hour to sometimes twenty-four hours. Scout bees are dispatched to search for a new home for the swarm while the queen and remaining bees await their return. The swarm will fly en masse to the new home described by the most convincing scout bee. The new home may consist of a hollow in a tree, a vacant beehive, an abandoned water heater, a cavity in a wall of a structure, or any other void that meets the specifications of the scout bees.
Identification
If a property owner suspects that a honey bee colony has entered the wall of a structure, he/she should attempt to confirm the insects are indeed honey bees. Other possible insects that might invade the wall of structures are carpenter bees, yellow jackets, or European hornets. Honey bees vary in color from yellow to black, have black or brown bands across the abdomen, and are much smaller than a carpenter bee. Honey bees are about 2/3 of an inch long and the body is covered with setae or hair. The foraging honey bees will have pollen baskets on each hind leg which will be often loaded with a ball of yellow or dark green pollen. The honey bee is the only stinging insect that can normally overwinter as a colony inside the wall of a structure in South Carolina.
The carpenter bee can be identified by having bright yellow, orange or white hairs on the thorax and a black shiny abdomen on the dorsal side. Carpenter bees are robust, heavy bodied bees that range 3/4 to 1 inch in length. These insects bore half inch wide holes that appear to be perfectly round on exterior wooden surfaces.
Yellow jackets lack the dense body hair that are found on carpenter bees and honey bees. The foraging yellow jackets do not have the pollen baskets on the hind legs. The yellow jacket is about .5 inch long and the abdomen is characterized by having alternating yellow and black bands. European hornets are much larger (1.5 inches long) than honey bees and sometimes establish colonies inside structural walls.
NOTE: The property owner must first decide whether to seek out a professional to do the job. Some beekeepers have much experience in honey bee removals and will remove the bees for a fee, but sometimes a carpenter will be needed to assist in reconstructing the wall. If you do not know a local beekeeper, you should call your Clemson University Cooperative Extension Office which may have a listing of local beekeepers. Also, some pest control companies have employees who are trained in bee removal.
Removal From Walls
The honey bee colony should be salvaged alive if possible, but sometimes this not practical. Honey bee swarms that have recently entered the wall of a structure say for a day or so can often be exterminated by injecting a pesticide recommended for bee control into the cavity. The bees should not have had time to construct a significant amount of comb, produce much brood, or store much honey unless a very strong nectar flow is in progress. Remember that if the bees have stored much honey in the wall and you kill the colony with a pesticide, the honey will be contaminated with the pesticide. Foraging bees from another nearby managed or feral bee colony will be attracted to the honey in the wall and may cause other unnecessary colony demise. To avoid this problem, caulk or place window screening on all potential entrances to the wall cavity such as knot holes and cracks in siding. This will prevent another swarm from entering the same cavity the next swarm season, too. A foul odor is to be expected for several weeks in the vicinity of the decaying bees if removal is not conducted.
Once the bees have setup housekeeping for more than a few days, the job often becomes more difficult. Sometimes, much comb (beeswax), brood, and honey are stored in the wall of a structure. Simply injecting a pesticide in the wall to kill the bees and leaving is risky. The comb will attract wax moths and mice for nesting sites. The honey will attract ants and other insects and may ooze through the wall or ceiling when comb melts during hot weather causing extensive damage.
A term called "neutralization" is necessary to get the job done right and avoid problems down the road. Neutralization involves the complete physical removal of all bees (dead or alive), comb, and honey from the wall following extermination to prevent future infestations. The removal process should be scheduled when no humans or pets are in the immediate area. A good wash down of the cavity with soapy water is recommended to remove all odors of the previous colony. If possible, it is a good idea to leave the void area open for a couple of weeks to allow drying and dissipation of colony odors. Filling the void prior to wall closure with spray foam insulation or fiberglass batting will prevent the space from re-colonization.
Depending on the exterior wall construction, the neutralization process may have to be conducted inside the structure, although exterior wall removal is preferred in most cases. The dimensions of the wall space occupied by the bee colony can normally be investigated in late evening by careful heat and noise observations. A stethoscope is a handy device to have when inspecting the size of the colony. An outline of the colony can be identified by carefully listening for a drastic decrease in the buzzing sound created by the bees. A light tap on the wall surface with a screw driver or hammer will elevate the noise intensity.
| Bee working supplies | Tools for structural work |
|---|---|
| Veil | Ladders |
| Bee suit | Hammer and nails |
| Bee working gloves | Crow bar |
| Hive tool | Aluminum cutters |
| Smoker | Saw (skill + cord, hand, chain+fuel) |
| Smoker fuel | Tin foil for sealing holes |
| Matches | Scaffold material for hive suspension |
| Hive body | Wire funnel |
| Screen hive entrance sealer | Staple gun |
| Flashlight | First aid kit |
| Stethoscope | Fire extinguisher |
| Knife | |
| Cutting/framing tray | |
| Wire or string | |
| 5 gal. buckets w/covers | |
| Bee brush | |
| Dust pan | |
| Soapy water | |
| Bee vacuum | |
| Sting kit or Epi-pen |
Most of the equipment listed above can be purchased at hardware stores, but the specialty equipment for working around honey bees cannot be found locally in many areas of our state. There are a few local South Carolina beekeeping supply dealers that can be found in the yellow pages or you may ask a beekeeper for references.
Removal of established bee colonies from structure walls can be made more pleasant by scheduling the job during the time of year when bee populations are low, a minimum of stored honey is present and the bees are less defensive. Early spring before the nectar flows have begun is the best time of year for bee removals in South Carolina. Another preferred time of year is late fall or on a mild winter day.
Another suggestion for bee removal from a structural wall is to remove parts of the exterior wall and disrupt the colony during extremely cold temperatures. Honey bees cannot fly during freezing weather and the bees will normally die from exposure. The neutralization process can be completed much quicker during cold temperatures. Remember-- although bees might be unable to fly in cold weather, they can sometimes crawl and sting.
So, you should observe the same safety precautions when working in cold weather.
Removal By Trapping
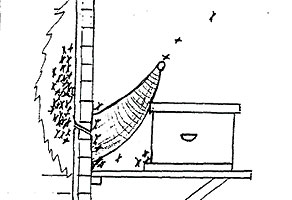
illustration of honey bee removal trap
Exterior stucco, brick, or cement walls make normal removal impossible, especially if interior wall accessibility is not an option. Trapping bees out of the wall with a "one-way bee escape removal" is recommended if a property owner is not in a hurry to have the colony removed. The process will take about 2-3 months and sometimes it is not successful unless careful attention to detail is followed. The comb will remain in the wall and will attract another swarm in the future unless preventive measures are taken.
The cone shaped one-way bee escape is constructed of window screen with the large end fastened over the primary bee entrance. It is imperative that all other cracks or holes leading to the bee colony be sealed off or your efforts will be unsuccessful. A hive body with a new queen bee inside is placed on the platform with the entrance as close to the primary entrance as possible. Returning foraging bees will fly to the base of the cone shaped bee escape and will be unable to reenter the wall. Eventually, the foraging bees will successfully gain entrance to the adjacent hive. Periodic checks to make sure the bees have not gained entry into the wall are necessary. As the colony in the wall weakens, the colony in the hive body will strengthen at the expense of the parent colony. The queen in the parent colony will not normally abandon her brood so a non-residual pesticide or CO2 should be injected into the wall to kill her and the remaining bees. Make sure the fumigant used does not leave a toxic residue. After a few days, the cone escape can be removed and the bees from the new hive will enter the wall and remove the remaining honey. All possible entry sites must be sealed or plugged to prevent re-colonization by future swarms. Filling the void with an expanding foam type of insulation is highly recommended.
Unfortunately, this trapping procedure requires many visits to the site to finish the job. The comb left behind in the wall will be highly attractive to scout bees in the future , therefore the structure owner should make annual inspections of the wall and refill any cracks or holes leading to the cavity.
Safety Around Honey Bees. One percent of the human population is allergic to bee stings. These individuals should take every precaution to avoid bee stings because one sting can be immediately life threatening. An available prescription sting kit is highly recommended for these individuals during outside activities.
WARNING: Honey bee removal from structures is not a job for a person who has never worked around stinging insects or even a novice beekeeper. Sometimes, this work is done while standing on a ladder with hundreds of angry bees trying to protect their home. Bee removal can be unnerving to even the experienced beekeeper and lead to accidents.
Prepared by William Michael Hood, Extension Entomologist/ Associate Professor, Department of Entomology, Soils, and Plant Sciences, Clemson University.
Providing Leadership in Environmental Entomology
Department of Entomology, Soils, and Plant Sciences, 114 Long Hall, Clemson, SC, 29634-0315, 864-656-3111
This information is supplied with the understanding that no discrimination is intended and no endorsement by the Clemson University Cooperative Extension Service is implied. Brand names of pesticides are given as a convenience and are neither an endorsement nor guarantee of the product nor a suggestion that similar products are not effective. Use pesticides only according to the directions on the label. Follow all directions, precautions and restrictions that are listed. EIIS/AP-3 (New 09/1998).
Honey Bee Tracheal Mite
Tracheal mites, parasites of the honey bee, were first discovered in South Carolina in the Mid-1980's. The mite has since spread throughout all regions of our state, leaving behind thousands of dead bee colonies. The tracheal mite is suspected of playing a major role in causing excessive statewide colony losses in recent years with losses reaching 80%.
History
Tracheal Mites (Acarapis woodi) were first discovered in 1919 on the Isle of Wight in the English Channel and were originally believed to be the cause of Isle of Wight disease which caused great colony losses. The Federal Honey Bee Act of 1922 was passed primarily to prevent spread of the mites into the United States.
In 1980, tracheal mites were discovered in Mexico, about 200 miles from the Southern U.S. border. USDA, APHIS, in cooperation with the Bioenvironmental Bee Lab at Beltsville, conducted a national survey for tracheal mites from 1980-82. Mite survey results were negative with samples collected from 4,400 apiaries.
On July 3, 1984, tracheal mites were first detected in the United States from bees sampled from a commercial beekeeping operation in Weslaco, Texas. Mites spread quickly throughout the U.S. by the movement of migratory beekeepers, sale of queens, packages and nucs.
Economic Impact
Much confusion exists in the U.S. as to the economic importance of this pest. Bee scientists located in Weslaco, Texas where the mite was first discovered have a difficult time maintaining mite levels high enough to conduct research. Florida beekeepers report minimum losses caused by the tracheal mite while other states report extreme devastation attributed to this parasitic mite.
In South Carolina, high colony losses were reported in the spring of 1989-90 although sufficient food stores were available. Examination of dead or weakened colonies yielded the presence of tracheal mites in most cases. Whether the mites were directly or indirectly responsible for colony deaths, many beekeepers became discouraged because of excessive colony losses and are no longer keeping bees.
Bee management costs have increased for most beekeepers due to having to replace dead colonies and purchase pesticides for tracheal mite control. These increased costs will have to be passed on to fruit and vegetable growers who rent hives for crop pollination. Increased management costs and rental fees, reduced honey production and pollination, and discouragement experienced by many beekeepers have resulted in a negative economic impact caused by tracheal mites in South Carolina.
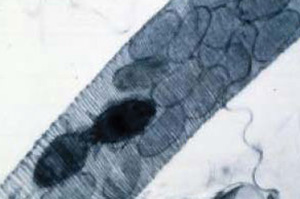
Tracheal mite adults and eggs inside honey bee trachea
Biology
Tracheal mites are microscopic in size (as long as 1.5 times the diameter of a human hair) and spend their entire life cycle within the tracheae (breathing tubes) of adult honey bees. Female mites lay single eggs in the bees respiratory tract where the developmental cycle occurs. Each female mite lays five to seven eggs 3 or 4 days after entering the tracheae and the eggs require three to four days to hatch. Adult mites (5-9 days old) emerge from their host tracheae in search of a new host.
Within 24 hours after the worker bees emerge from their cells, female mites collect within their tracheae. Studies have found that highest number of mites are found in 11-12 day old adult bees, and mite levels decline over 21 days old.
Adult mites penetrate the tracheal wall with their piercing mouthparts and feed on bee blood. Normal thoracic tracheae are clear, colorless, or pale amber in color. Severely infested tracheae are darkened with crust-like lesions and may appear black. Numerous mites in varying stages of development and mite debris inside the tracheae are thought to reduce capacity for airflow.

Adult female tracheal mite suspended from honey bee hair.
(400X) Courtesy of Tom Webster.
Bee scientists have discovered a connection between tracheal mite infested bees and flight muscle damage. Flight muscles of mite infested crawler bees are degenerated which affects the ability to fly. Also, blood tests from mite infested bees show a higher than normal bacterial count.
Drifting bees between hives, and swarms produced from infested colonies are ways mites are naturally spread within an apiary and between apiaries. Mites are spread within a colony by bee contact. Mature female mites exit the bee trachea and climb to the tip of a body hair. Upon contact with a newly emerged bee, the mite transfers to the hairs of the new host and enters the trachea where it will complete it life cycle. If the mite fails to locate a new host within 24 hours, it will die.
The tracheal mite population may vary seasonally. During the period of maximum bee population, the number of bees with mites is reduced.
Symptoms
No one symptom characterizes a tracheal mite infested colony. Tracheal mites are believed to shorten the life span of adult bees, affect flight activity, and result in a large number of crawling bees that are unable to fly. The wings of mite infested bees are often disjointed with the hind wing projecting 90° from axis of the body. However, absence of these symptoms does not necessarily indicate freedom of mites.
Infected colonies may not develop normally and may exhibit symptoms of dysentery and an excessive swarming tendency. Often, however, severely infested colonies typically appear normal until their death during the winter. Colonies are most affected during winter confinement and early spring like a stress disease. Mite infestations are at a maximum in the spring when the population is comprised of primarily older bees.
Host Susceptibility
The susceptibility of worker honey bees to tracheal mites diminishes rapidly with age and bees over nine days old rarely become infested. Studies have shown that adult female mites transfer preferentially to young bees less than 4 days old, and newly emerged bees, less than 24 hours old, are especially attractive. Research is being conducted to determine variations in tracheal mite susceptibility among U.S. bee stocks.
Diagnosis
Positive diagnosis can be made only by microscopic examination of honey bee tracheae. In sampling for this mite, one should try to collect either bees that may be crawling near the hive entrance or bees at the entrance as they are leaving or returning to the hive (50 bees per colony sampled). These bees should be placed in 70% ethyl or methyl alcohol as soon as they are collected. Do not collect bees that have been dead for an unknown period because they are less than ideal for diagnosis.
For dissection, each bee should be grasped between your thumb and forefinger, remove front pair of legs and head by pushing them off with a scalpel or razor blade in a downward and forward motion, cut thin transverse section of the prothorax containing the major tracheal trunks with a sharp razor blade, soak disc section overnight with 8% solution of Potassium Hydroxide in water to dissolve muscle tissue, and examine disc sections with a microscope (Minimum 40X. Infested tracheae have various stages of mites and are usually discolored and darkened.
Control
Menthol. Menthol crystals provide a fumigant action that kills tracheal mites without harming the bees at certain temperatures. The crystals start to evaporate about 70°F and will melt to a liquid at about 102-105°F. The vapors are heavier than air and best evaporation rate occurs between 80° and 85°F.
Menthol crystals were registered as a pesticide for tracheal mite control in the U.S. in January, 1989. Varying degrees of mite control have been achieved with menthol treatments in the U.S. Effectiveness of the product is dependent upon temperature, formulation (crystals or pellets), dosage, colony, size, condition of equipment, position, within the hive and exposure time.
Menthol should be used during a non-nectar flow period and after surplus honey has been removed to prevent menthol-flavored honey. Fifty grams (108 oz) of crystals in 7-inch square porous packet should be placed on top frame bars in the brood chamber if the temperature is below 80°F. If the temperature is 80°F or greater, the packet should be placed on the bottom board. Replace packets in the hive with fresh material as needed to maintain menthol in the hive for the specified treatment period.
Remove all menthol packets from the hive in 10-12 weeks following the initial treatment. Remove all menthol packets at least one month prior to a nectar flow to prevent contaminating marketable honey. Menthol crystals may reduce brood-rearing and affect clustering behavior if left on the colony beyond the recommended treatment period.
A combination of menthol crystals and vegetable shortening-sugar patty treatments should give optimum mite control.
In South Carolina, many beekeepers have reported excellent mite control with menthol packets placed on the bottom board in August and removed in November. Results may vary depending on local conditions. A yearly fall treatment should reduce the mite population and allow good over-wintering and good spring buildup.
Vegetable Shortening-Sugar Patty. A vegetable shortening-sugar patty treatment has been shown to provide good tracheal mite control. This treatment is thought to disrupt the tracheal mite life cycle by reducing the ability of the female mite to detect young bees as hosts.
The patties are made of two parts granulated sugar and one part vegetable shortening. A baseball size patty should be flattened and placed on the top frame bars in the brood chamber. The patty can be placed on wax paper over the top frame bars, but this is optional. Patties should be placed in colonies during brood rearing periods. A fall or early spring treatment or both is recommended.
Tracheal mites require their host to survive. Empty equipment that has been free of live bees for one week may be reused without mite treatment.
Precautionary Statement
In order to protect people and the environment, pesticides should be used safely. This is everyone’s responsibility, especially the user. Read and follow label directions carefully before you buy, mix, apply, store or dispose of a pesticide. It is a violation of State and Federal Laws to use pesticides in a manner inconsistent with its label.
Disclaimer Statement
Pesticides recommended in this publication were registered for the prescribed uses when printed. Pesticide registrations are continuously being reviewed. Should registration of a recommended pesticide be canceled, Clemson University would no longer recommend it.
References
Shimanuki, H. and D. Knox. 1991. Diagnosis of Honey Bee Disease. USDA, Ag. Handbook No. A-690, 5 p.
Collison, C.H. 1990. Tracheal Mites Past-Present- Future. ESA Journal Vol. 17, No. 3 pp. 5-6.
Skinner, J. 1991. Tracheal Mites in Tennessee. E & PP Info 952 p.
Prepared by William Michael Hood, Extension Entomologist/ Associate Professor, Department of Entomology, Soils, and Plant Sciences, Clemson University.
Providing Leadership in Environmental Entomology
Department of Entomology, Soils, and Plant Sciences, 114 Long Hall, Clemson, SC, 29634-0315, 864-656-3111
This information is supplied with the understanding that no discrimination is intended and no endorsement by the Clemson University Cooperative Extension Service is implied. Brand names of pesticides are given as a convenience and are neither an endorsement nor guarantee of the product nor a suggestion that similar products are not effective. Use pesticides only according to the directions on the label. Follow all directions, precautions and restrictions that are listed. EIIS/AP-4 (New 01/2000).
